Molecule are positively charged ions. Click to see full answer.

Which Of The Following Compounds Does Not Follow The Octet Rule Youtube
A cation has fewer electrons than protons.
. Then does h20 follow the octet rule. Hydrogens nearest noble gas helium has a filled shell with only two electrons. However many atoms below atomic number 20 often form compounds that do not follow the octet rule.
Oxygen has 6 valence electrons the bonds should be 8-62 bonds. So O2 does not satisfy the octet rule because as we know octet rule states that an atom has to have 8 e- in the outer shell. The boron shares its.
A NF3 b CF4 c SF4 d PH3 e HCl I know I can eliminate A and B because nitrogen and carbon follow the octet rule. Hydrogen Lithium Phosphorus Sulfur. There are also a.
Which element does not follow the octet rule. Elements like hydrogen lithium helium do not obey the octet rule. Exceptions to the octet rule fall into one of three categories.
An octet corresponds to an electron configuration ending with s2p6. So we need 2 more covalent bonds to form an octet. I know I can eliminate E because the total.
The octet rule states that atoms below atomic number 20 tend to combine so that they. Exceptions to the Octet Rule. Some elements that disobey the octet rule include.
Which statement best explains why hydrogen forms ions that do not follow the octet rule. As such your oxygen atom in HOH is actually following the octet rule as in there are eight valence positions around the oxygen atom six from the p-shell positions and two from the s-shell and the covalent bonding with 2 hydrogen atoms plus the 6 electrons supplied from oxygen yield 6 1 1 8 which is the. Key Takeaways The Octet Rule and Its Exceptions.
Chlorine and sulfur will not strictly follow the octet rule. The octet rule is described. A compound or element containing two or more atoms joined together with covalent bonds.
1 an incomplete octet 2 odd-electron molecules and 3 an expanded octet. There are many compounds that do not follow octet rulethe rule that suggest that every element gets stability by acquiring eight electrons in outermost or valance shell but here I will quote the example of PCl5phosphorous penta chlorideIn PCl5 P atom has 5 electrons in outermost or valance shellso in order to complete its octetit must form 3 bonds as in case of PCl3 but it. Another exception of the octet rule is transition elements.
What element does not follow the octet rule. Which one of the following compounds does not follow the octet rule. As the saying goes all rules are made to be broken.
When it comes to the octet rule that is true. They can only lose or gain one electron to become stable due to which they follow the octet rule. Explaination Expanded octet can be seen in atoms that have vacant orbital this is an essential requireme View the full answer.
Boron hes how Other notable exceptions are aluminum and boron which can function well with six valence electrons.

Which Of The Following Does Not Follow The Octet Rule Youtube

Ppt Exceptions To The Octet Rule Powerpoint Presentation Free Download Id 3528141

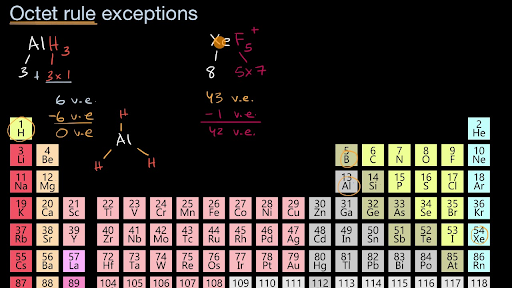
0 Comments